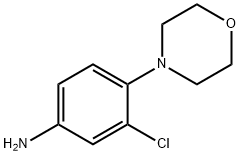
3-CHLORO-4-MORPHOLINOANILINE synthesis
- Product Name:3-CHLORO-4-MORPHOLINOANILINE
- CAS Number:55048-24-3
- Molecular formula:C10H13ClN2O
- Molecular Weight:212.68
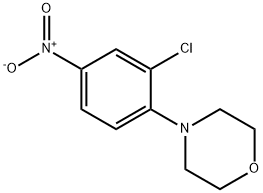
55435-71-7
40 suppliers
$14.19/250mg

55048-24-3
63 suppliers
$45.00/250 mg
Yield: 96%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 110; for 0.75 h;Inert atmosphere;Sealed tube;
Steps:
I.2 Scheme 58, Step 2
In a 20 mL Biotage microwave vial, a stir bar, 4-(2-chloro-4-nitrophenyl)morpholine 168-A (500 mg, 2.061 mmol), iron (575 mg, 10.30 mmol), and ammonium chloride (220 mg, 4.12 mmol), were added. The vial was sealed, degassed with Ar for 15 min, and degassed EtOH:H2O (5.15 mL: 5.15 mL, 1:1 v/v) injected. The mixture was heated for 0.5 h at 110° C. in an oil bath. Upon cooling, the mixture was diluted with EtOAc, filtered through celite, transferred to a separatory funnel and 10% NaOH was added. The aq layer was extracted with ethyl acetate and the pooled organics were washed with saturated brine and dried with Na2SO4. Solvent was concentrated, redissolved in DCM, dryloaded onto silica and chromatographed on a 12 g silica column, (DCM/MeOH, 0-5%), affording 3-chloro-4-morpholinoaniline 168-B as a pink solid (420 mg, 1.975 mmol, 96% yield, 5% MeOH). 1H NMR (CDCl3) δ: 6.89 (d, J=8.5 Hz, 1H), 6.74 (d, J=2.7 Hz, 1H), 6.56 (dd, J=8.5, 2.7 Hz, 1H), 3.90-3.82 (m, 4H), 3.61 (s, 2H), 2.98-2.91 (m, 4H). 13C NMR (CDCl3) δ: 143.20, 140.65, 129.87, 121.31, 117.10, 114.17, 67.30, 52.28.
References:
Arizona Board of Regents on Behalf of the University of Arizona;Hulme, Christopher;Foley, Christopher US2020/39989, 2020, A1 Location in patent:Paragraph 1041-1042
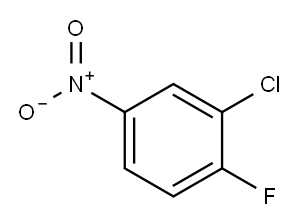
350-30-1
316 suppliers
$14.00/1g

55048-24-3
63 suppliers
$45.00/250 mg