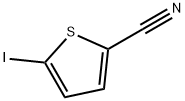
5-Iodo-2-thiophenecarbonitrile synthesis
- Product Name:5-Iodo-2-thiophenecarbonitrile
- CAS Number:18945-81-8
- Molecular formula:C5H2INS
- Molecular Weight:235.05
1003-31-2
295 suppliers
$6.00/5g

18945-81-8
38 suppliers
inquiry
Yield:18945-81-8 88%
Reaction Conditions:
Stage #1: thiophene-2-carbonitrilewith 2,2,6,6-tetramethylpiperidinylmagnesium chloride lithium chloride complex in tetrahydrofuran at 25; for 2 h;
Stage #2: with iodine in tetrahydrofuran at 25; for 1 h;
Steps:
Typical procedure for metalation with TMPMgCl.LiCl followed by the reaction withelectrophiles (iodine, PhSeSePh and PhSSPh)
General procedure: In a flame-dried flask flushed with nitrogen, the heterocyclic nitrile (1 mmol) wasdissolved in THF (1.5 mL). TMPMgCl·LiCl (1.0 M in THF, 1.8 mL, 1.8 mmol) wasadded dropwise at 25°C and the mixture was stirred for 2h (for substrate 1a, metalationwas best performed in 1 h). The completion of the metalation was checked by GC analysisof reaction aliquots quenched with a solution of I2 in dry THF. After that, a solution ofthe electrophile (2 mmol) in THF (2 mL) was added dropwise at 0°C and the reactionstirred for 1 hour at 25°C. The reaction mixture was quenched with sat. aq. NaHSO3 orNH4Cl solution (5 mL), extracted with ethyl acetate (3x15 mL) and dried over anhydrousMgSO4. After the filtration, solvent was evaporated in vacuo.
References:
Dos Santos, Fernanda M.;Batista, Jo?o H. C.;Vessecchi, Ricardo;Clososki, Giuliano C. [Synlett,2015,vol. 26,# 20,art. no. ST-2015-S0611-C,p. 2795 - 2800] Location in patent:supporting information