Abemaciclib synthesis
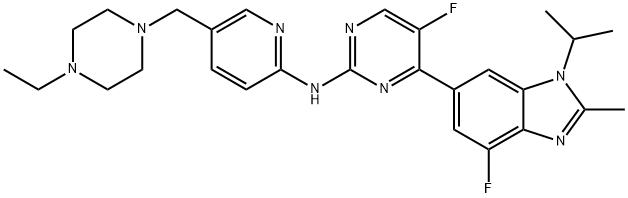
- Product Name:Abemaciclib
- CAS Number:1231929-97-7
- Molecular formula:C27H32F2N8
- Molecular Weight:506.59

Reference:Chan ED. Combination of anti-human VEGFR2 antibody and abemaciclib for treatment of non-small cell lung cancer. WO 2015130540 (2015).

1180132-17-5
196 suppliers
$25.00/250mg
![6-Bromo-4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazole](/CAS/GIF/1231930-33-8.gif)
1231930-33-8
199 suppliers
$13.00/100mg

1231929-97-7
250 suppliers
$29.00/1mg
Yield:1231929-97-7 82.85%
Reaction Conditions:
with palladium diacetate;potassium carbonate;4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene at 98 - 100;Inert atmosphere;
Steps:
30 Example 30: Preparation of abemaciclib
Argon/Nitrogen was bubbled into a mixture of 6-bromo-4-fluoro-1-isopropyl-2-methyl-1H- benzoimidazole (10 g, 0.0309 mol), 5-(4-ethyl-piperazin-1-ylmethyl)-pyridin-2-ylamine (6.890.0312 mol), potassium carbonate (8.65 g), and xantphos (1.07 g, 0.0018 mole) in tert-amyl alcohol (50 mL). Palladium acetate (0.2 g, 0.00089 mole) was added and the reaction mass was heated to 98-100 °C for 2-4 hours. The completion of the reaction was monitored by TLC/HPLC. The reaction mass was cooled to 30-35 °C., then diluted with dichloromethane (80 mL) and water (30 mL) and filtered through a HYFLO bed. The compound was extracted from theorganic layer using hydrochloric acid diluted in water (1:1) (2 x 30 mL). The pH of the combined aqueous layers was adjusted to 11-12 with a sodium hydroxide solution and the compound was extracted using dichloromethane. The organic layer was treated with N-acetyl-L-cysteine and the pH of the solution was adjusted to 11.0-12.0 with a sodium hydroxide solution. The mixture was stirred for 30 minutes at 30-35 °C and the layers were separated. The organic layer was washedwith water and distilled under vacuum at 40-45 °C. Acetone (100 mL) was charged to the residue and the temperature was raised to reflux for 30 minutes, and then cooled to 30-35 °C. The mixture was filtered and the solid product was washed with acetone and dried at 50-55 °C to give 13 g (82.85%) of abemaciclib with purity of 99.79%.
References:
MYLAN LABORATORIES LIMITED;JETTI, Ramakoteswara Rao;INDUKURI, Anjaneyaraju;BOMMAREDDY, Aggi Ramireddy;SRINIVASARAO, Attanti Veera Venkata;JEBARAJ, Rathinapandian;CHANDUPATLA, Shivakumar;BATHARAJU, Ramesh;KUNAMNENI, Sunil WO2019/102492, 2019, A1 Location in patent:Page/Page column 42

1180132-17-5
196 suppliers
$25.00/250mg

1231930-42-9
138 suppliers
$12.00/100mg

1231929-97-7
250 suppliers
$29.00/1mg

1231930-37-2
127 suppliers
$20.00/100mg

1231929-97-7
250 suppliers
$29.00/1mg

1118-69-0
93 suppliers
$59.00/25g

1231929-97-7
250 suppliers
$29.00/1mg

67567-26-4
340 suppliers
$5.00/1g

1231929-97-7
250 suppliers
$29.00/1mg




