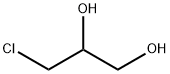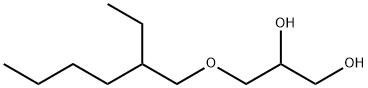
Ethylhexylglycerin synthesis
- Product Name:Ethylhexylglycerin
- CAS Number:70445-33-9
- Molecular formula:C11H24O3
- Molecular Weight:204.31
Yield:70445-33-9 92.4%
Reaction Conditions:
with sodium hydroxide at 90; for 0.666667 h;Temperature;Reagent/catalyst;
Steps:
1-4 Example 4
Stir the sodium hydroxide and isooctanol in the pre-mixing tank 1 according to 1.5:1 (molar ratio), and then enter the first oil bath raw material storage tank 4 to preheat to 90°C, and add 3-chloro-1,2- Propylene glycol is preheated to 90°C in the second oil bath raw material storage tank 8.The above-mentioned two preheated raw materials were pumped into the micromixer 12 according to the flow volume ratio of 1:1.3, mixed, and then entered into the microchannel reactor 13 for reaction. The diameter of the microchannel reactor 13 was 2.0mm, and the reaction temperature was 90. , The reaction time is 40 minutes, and the crude ethylhexyl glycerol is obtained. The content of ethylhexyl glycerol in online GC analysis is 94.6%.Adjust the PH value of the crude product to 7 with hydrochloric acid solution, lower it to room temperature, separate the product from oil and water with a separatory funnel, drain the water phase, wash the organic phase twice with water, separate the organic phase, and pass the molecular distillation equipment in a vacuum The temperature is 40-50Pa, and 138±3°C fractions are collected to obtain ethylhexylglycerol as a colorless and transparent liquid. According to gas chromatography analysis, the purity of ethylhexylglycerol is 99.7%, and the yield is 92.4%.
References:
CN112661614,2021,A Location in patent:Paragraph 0026-0033
2461-15-6
217 suppliers
$30.00/25mL

70445-33-9
365 suppliers
$10.00/5g

123-05-7
125 suppliers
$10.00/5mL
56-81-5
1623 suppliers
$5.00/25g
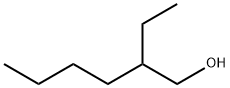
104-76-7
656 suppliers
$5.00/10g
![1,3-bis[(2-ethylhexyl)oxy]propan-2-ol](/CAS/GIF/59068-03-0.gif)
59068-03-0
4 suppliers
inquiry
![1,3-Propanediol, 2-[(2-ethylhexyl)oxy]-](/CAS/20210305/GIF/1027549-81-0.gif)
1027549-81-0
0 suppliers
inquiry
![2,3-bis[(2-ethylhexyl)oxy]propan-1-ol](/CAS/GIF/59068-04-1.gif)
59068-04-1
1 suppliers
inquiry

70445-33-9
365 suppliers
$10.00/5g
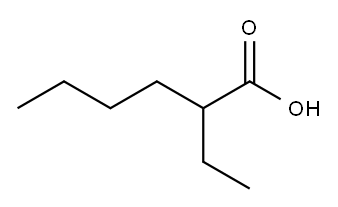
149-57-5
511 suppliers
$5.00/25g

56-81-5
1623 suppliers
$5.00/25g

70445-33-9
365 suppliers
$10.00/5g

104-76-7
656 suppliers
$5.00/10g
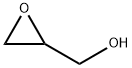
556-52-5
250 suppliers
$17.67/1gm:

70445-33-9
365 suppliers
$10.00/5g