
Ozagrel synthesis
- Product Name:Ozagrel
- CAS Number:78712-80-8
- Molecular formula:C15H16N2O2
- Molecular Weight:256.3
Yield:78712-80-8 96.4%
Reaction Conditions:
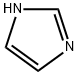
Stage #1: 1H-imidazolewith potassium hydroxide in acetonitrile at 20; for 0.0166667 h;
Stage #2: ethyl (E)-4-(bromomethyl)cinnamate in acetonitrile at 0 - 5; for 3 h;Temperature;Solvent;
Steps:
3; 5 Implementation example 3
Add 340g (5.0mol, 2.0eq) of imidazole, 280g (5.0mol, 2.0eq) of potassium hydroxide, 1.8L of acetonitrile to a 10L reaction flask, stir and react at room temperature for 1h, the system becomes orange-red liquid, and the temperature is controlled at 05 Continue to stir the reaction for 30 min at °C; dissolve 670 g (2.5 mol, 1.0 eq) of ethyl 4-bromomethyl cinnamate in 3.0L of acetonitrile, stir to make it clear, and add the ethyl 4-bromomethyl cinnamate in batches. The acetonitrile solution was gradually added dropwise to the imidazole/potassium hydroxide reaction system through the dropping funnel. After the addition, the reaction was continued at 05 for 3h, and the reaction was monitored by TLC (developing solvent: ethyl acetate/isopropanol/water/ammonia =7ml/14ml/5ml/0.6ml, UV254nm) until the raw material disappears and stop the reaction; add acetic acid to adjust the pH to 6-7, the reaction solution is concentrated under reduced pressure, and acetonitrile is recovered. Crystallize, filter, and dry the filter cake at 60°C to a constant weight to obtain a white flocculent solid, weighing 615 g, with a yield of 96.4%. The purity is 98.20%.
References:
CN113493416,2021,A Location in patent:Paragraph 0020; 0040-0041; 0044-0045
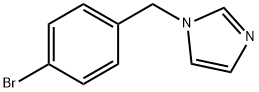
72459-46-2
62 suppliers
$53.00/250mg
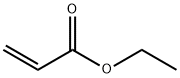
140-88-5
511 suppliers
$12.00/100ml

78712-80-8
20 suppliers
inquiry

64-17-5
699 suppliers
$10.00/50g
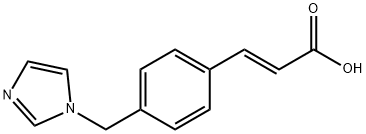
82571-53-7
238 suppliers
$26.00/5mg

78712-80-8
20 suppliers
inquiry
![(E)-3-[p-Bromomethylphenyl]acrylic acid ethyl ester](/CAS/GIF/78712-67-1.gif)