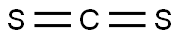Phenethyl isothiocyanate synthesis
- Product Name:Phenethyl isothiocyanate
- CAS Number:2257-09-2
- Molecular formula:C9H9NS
- Molecular Weight:163.24
Yield:2257-09-2 94%
Reaction Conditions:
Stage #1:carbon disulfide;phenethylamine with triethylamine in tetrahydrofuran at 0 - 20; for 0.5 h;Inert atmosphere;
Stage #2: with acetyl chloride in tetrahydrofuran at 0 - 20;Inert atmosphere;Reagent/catalyst;
Steps:
3.1. General Procedure Exemplified by the Synthesis of PEITC
To a solution of phenethylamine (200 mg, 1.65 mmol) and Et3N (0.64 mL, 4.95 mmol) in anhydrousTHF (2.5 mL) cooled with an ice bath, a solution of CS2 (0.12 mL, 1.98 mmol) was slowly dropped in.The reaction solution was stirred at room temperature for 0.5 h, after which AcCl (0.14 mL, 1.98 mmol)was dropped in at 0 °C, and after 5 min the mixture was warmed to room temperature for 15-30 min.When the starting amine was finished, as verified by checking thin layer chromatography (T.L.C.),1MHCl (aq., 2 mL) was added to quench the reaction. The solution was extracted by EtOAc three times.All organic phases were combined and washed with brine, dried over Na2SO4, and finally filtered andconcentrated under reduced pressure. The residue was purified by column chromatography on silicagel (EtOAc/petroleum = 1/1) to provide phenethyl isothiocyanate (PEITC) (253 mg, 94%).3.2. Characterization of Synthetic IsothiocyanatesPhenethyl isothiocyanate/PEITC. Yellow liquid, yield: 94%, 1H-NMR (400 Hz, CDCl3): 7.25-7.40(m, 5H), 3.75 (t, 2H, J = 6.8 Hz), 3.02 (t, 2H, J = 6.8 Hz).
References:
Luo, Bingling;Wang, Jiankang;Li, Xiaobing;Lu, Wenhua;Yang, Jing;Hu, Yumin;Huang, Peng;Wen, Shijun [Molecules,2017,vol. 22,# 6,art. no. 773]
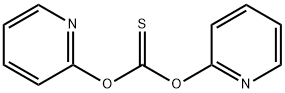
96989-50-3
108 suppliers
$48.00/250mg
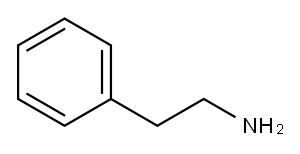
64-04-0
8 suppliers
$10.00/5mL

2257-09-2
186 suppliers
$31.00/5g