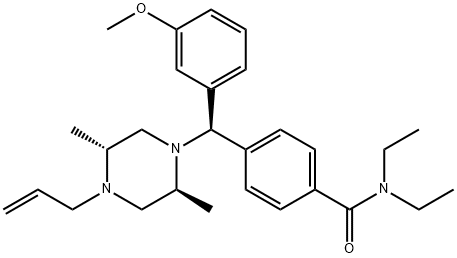
SNC 80 synthesis
- Product Name:SNC 80
- CAS Number:156727-74-1
- Molecular formula:C28H39N3O2
- Molecular Weight:449.63
58287-77-7
10 suppliers
inquiry

36282-40-3
92 suppliers
$88.40/100ml

155836-78-5
29 suppliers
$139.80/10 mg

156727-74-1
81 suppliers
$55.00/5mg
Yield: 35%
Reaction Conditions:
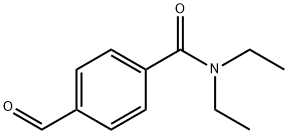
Stage #1:N,N-diethyl-4-formylbenzamide;(-)-(2R,5S)-1-allyl-2,5-dimethylpiperazine with 1,2,3-Benzotriazole in toluene for 2 - 3 h;Heating / reflux;
Stage #2:(3-methoxyphenyl)magnesium bromide at 0 - 20; for 1.5 h;
Stage #3: with water;ammonium chloride
Steps:
3
Benzotriazole (22.3 g, 187.5 mmol), 4-formyl-N,N-diethylbenzamide (38.5 g, 187.5 mmol, from Example 1), and (2R,5S)-l-allyl-2,5-dimethylpiperazine (28.9 g, 187.5 mmol, Chirotech EPO
References:
Location in patent:Page/Page column 23-24

186094-10-0
11 suppliers
$110.00/50mg

155836-78-5
29 suppliers
$139.80/10 mg

156727-74-1
81 suppliers
$55.00/5mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

82520-37-4
18 suppliers
$121.00/0.5g

156727-74-1
81 suppliers
$55.00/5mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

156727-74-1
81 suppliers
$55.00/5mg

156727-76-3
10 suppliers
$283.00/250mg

156727-74-1
81 suppliers
$55.00/5mg