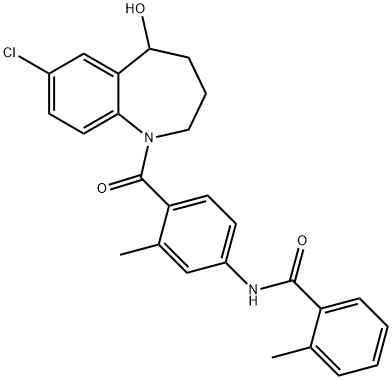
Tolvaptan synthesis
- Product Name:Tolvaptan
- CAS Number:150683-30-0
- Molecular formula:C26H25ClN2O3
- Molecular Weight:448.94

Cordero-Vargas, Alejandro; Quiclet-Sire, Beatrice; Zard, Samir Z. A flexible approach for the preparation of substituted benzazepines: Application to the synthesis of tolvaptan. Bioorganic & Medicinal Chemistry. Volume 14. Issue 18. Pages 6165-6173. 2006.
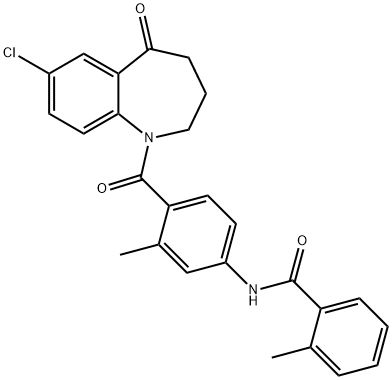
137973-76-3
144 suppliers
$59.00/2 mg

150683-30-0
344 suppliers
$25.00/1mg
Yield:150683-30-0 96%
Reaction Conditions:
with methanol;sodium tetrahydroborate at 0; for 2 h;
Steps:
1.7 (7) Preparation of N-[4-[(7-chloro-2,3,4,5-tetrahydro-5-hydroxy-1H-1-benzazepine-1-yl)carbonyl]-3-methylphenyl]-2-methylbenzamide
7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-1- benzazepine (19.0g, 42.51mmol) was dissolved in methanol (200ml) in a solvent, at 0 for Conditional batch of sodium borohydride (2.42g, 63.77mmol), after the addition was complete stirring was continued for 2h, TLC the reaction was complete. The reaction mixture was poured into water, extracted with methylene chloride, dried over anhydrous sodium sulfate, filtration and vacuum distillation of crude with methanol: petroleum ether (2: 1) and recrystallized to give a white solid tolvaptan (18.33g , 96.0%).
References:
Shanghai Tin Tsz Bio Valley Biological Engineering Co.;Li, Xinjuanzi;Li, Jianzhi;Ma, Xilai;Chi, Wangzhou;Liu, Hai;Hu, Xuhua;Zheng, Xiaoli;Di, Zhijun;Li, Jianxun CN105315169, 2016, A Location in patent:Paragraph 0170; 0211; 0212

160129-45-3
287 suppliers
$8.00/250mg

150683-30-0
344 suppliers
$25.00/1mg

137982-91-3
130 suppliers
inquiry

150683-30-0
344 suppliers
$25.00/1mg

30459-70-2
50 suppliers
inquiry

150683-30-0
344 suppliers
$25.00/1mg
5202-89-1
301 suppliers
$12.00/5g

150683-30-0
344 suppliers
$25.00/1mg








