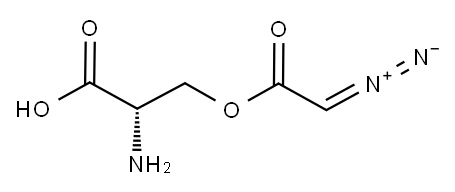
AZASERINE
- CAS No.
- 115-02-6
- Chemical Name:
- AZASERINE
- Synonyms
- azs;ci337;cl337;p-165;ci-337;nsc-742;cn15757;azaserin;AZASERINE;cn-15,757
- CBNumber:
- CB3388053
- Molecular Formula:
- C5H7N3O4
- Molecular Weight:
- 173.13
- MDL Number:
- MFCD00036802
- MOL File:
- 115-02-6.mol
- MSDS File:
- SDS
| Melting point | 146-162° (dec) |
|---|---|
| alpha | D27.5 -0.5° (c = 8.46% in H2O at pH 5.18) |
| Boiling point | 303.75°C (rough estimate) |
| Density | 1.5830 (rough estimate) |
| refractive index | 1.6190 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL, clear, yellow |
| form | lyophilized powder |
| pka | 8.55(at 25℃) |
| color | Light-yellow needles from EtOH (aq) |
| Merck | 13,902 |
| BRN | 1726602 |
| FDA UNII | 87299V3Q9W |
| Proposition 65 List | Azaserine |
| NCI Drug Dictionary | azaserine |
| IARC | 2B (Vol. 10, Sup 7) 1987 |
| EPA Substance Registry System | Azaserine (115-02-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H351 | |||||||||
| Precautionary statements | P201-P301+P310+P330 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 25-40 | |||||||||
| Safety Statements | 53-36/37/39-45 | |||||||||
| RIDADR | UN 3462 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | VT9625000 | |||||||||
| F | 10 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 in mice, rats (mg/kg/day): 150, 170 orally (Sternberg, Philips) | |||||||||
| NFPA 704 |
|
AZASERINE price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A4142 | Azaserine ≥98% (TLC) | 115-02-6 | 50mg | $264 | 2024-03-01 | Buy |
| Sigma-Aldrich | A1164 | Azaserine Hybri-Max?, γ-irradiated, 50x, lyophilized powder, BioXtra, suitable for hybridoma | 115-02-6 | 0.5MG | $46 | 2024-03-01 | Buy |
| Sigma-Aldrich | A1164 | Azaserine Hybri-Max | 115-02-6 | .5mg | $21.3 | 2021-12-16 | Buy |
| Cayman Chemical | 14834 | Azaserine ≥98% | 115-02-6 | 10mg | $37 | 2024-03-01 | Buy |
| Cayman Chemical | 14834 | Azaserine ≥98% | 115-02-6 | 25mg | $81 | 2024-03-01 | Buy |
AZASERINE Chemical Properties,Uses,Production
Chemical Properties
Light-yellow needles from EtOH.
Originator
Azaserine,TG International Chemical Co.
Uses
antineoplastic, amino acid antagonist
Uses
Reagent used to induce pancreatic cancer in experimental animal models.
Definition
ChEBI: A carboxylic ester resulting from the formal condensation of the carboxy group of diazoacetic acid with the alcoholic hydroxy group of L-serine. An antibiotic produced by a Streptomyces species.
Manufacturing Process
The azaserine is produced by microbiological synthesis using culture of
Streptoniyces fragili:
10 gallons of a nutrient medium having the following composition (%):glucose
1.0, soybean expeller oil meal 1.0, acid hydrolyzed casein 0.5, debittered
yeast 0.5, sodium chloride 0.5, water sufficient to make 100.0% is placed in a
30 gallon stainless steel fermenter, the pH adjusted to 7.5 with 6 N sodium
hydroxide solution and 0.1% calcium carbonate added. The medium is
sterilized by heating at 121°C for 30 min after which the pH of the medium is
6.85. The medium is cooled and inoculated with the spares from two fourteen
day old Moyer's sporulation agar slant cultures of Streptoniyces fragilis
suspended in 20 ml of sterile 0.01% castile soap solution. The culture mixture
is incubated at 27°C for 24 h during which time aeration is supplied through a
sparger at the rate of one volume of air per volume of medium per minute.
The incubated culture thus obtained is used to inoculate the main culture as
described below. 150 gallons of a medium having the following composition
(%): glucose 1.0, soybean expeller oil meal 1.0, acid hydrolyzed casein 0.5,
debittered yeast 0.5, sodium chloride 0.5, ammonium nitrate 0.25, water
sufficient to make 100.0 percent. 6 N sodium hydroxide solution-sufficient to
bring the pH to 7.5,calcium carbonate - (added after pH adjustment) is placed
in a 200-gallon stainless steel fermenter and sterilized by heating at 121°C for
30 min. The medium is cooled, inoculated with the 10-gallon culture of
Streptomyces fragilis prepared as described above, and incubated at 26°C for
44 h. During the incubation period air is supplied through a sparger at the
rate of 1.5-volumes of air per volume of medium per min and the mixture
stirred at the rate of 150 r.p.m. for the first 12 h and at 300 r.p.m. for the
final 32 h, 1.5 gallons of a sterilized mixture of crude lard and mineral oils
containing mono- and diglycerides being added as needed to control foaming.
The solid material present in the incubated fermentation mixture is removed
by filtration and the filter cake washed with water. The washings are combined
with the main filtrate and 110 gallons of this solution stirred with 2079.0 g of
activated carbon for about 1 h. The carbon is removed by filtration and the
filter cake washed with deionized water. The combined filtrate and washes
(136 gallons) are concentrated in vacuum to a volume of about 20 gallons.
Three volumes of acetone are added to the concentrate with stirring, and the
precipitate which forms removed by filtration and the filter cake washed with
75% aqueous acetone. The combined aqueous acetone filtrate and washings
is concentrated in vacuum to a volume of about 19.5 gallons, the concentrate
so obtained frozen and dried from the frozen state under high vacuum. 1.0 kg
of dry powder is extracted with one 10-liter portion of 90% (by volume)
ethanol followed by extraction with one 2-liter portion of the same solvent.
The combined extracts (about 12 L) are diluted with sufficient water to reduce
the ethanol concentration to 75% by volume, and this alcoholic solution
passed through an adsorption column prepared as described below.
3.0 kg of alumina are stirred with dilute hydrochloric acid so that the pH
remains constant at 7.7. The alumina is removed, washed with water and
activated by heating at 200°C for 4 h. The alumina is stirred with 75%
aqueous ethanol and packed into an adsorption column having a diameter of 4
inches. The total packed volume is approximately 3500 ml.
The alcoholic solution prepared above is added to the adsorption column at
the rate of 6 L/h and the percolate discorded. The column is washed with 35 L
of 75% ethanol (by volume), the washing discarded and the column finally
washed with 21 L of 50% ethanol. Some O-diazoacetyl-L-serine may be detected in the last wash solution. After the washing has been completed the
adsorbed O-diazoacetyl-L-serine is eluted from the adsorption column by
passing 17.5 L of distilled water through the column. The aqueous eluate is
concentrated and frozen and the concentrate dried from the frozen state
under high vacuum. The powder thus obtained, a O-diazoacetyl-L-serine
content of 5.8%.
500.0 g of the material assaying 5.8% O-diazoacetyl-L-serine is dissolved in
1,320 ml of water. A column of activated charcoal is prepared. A mixture of
2.0 kg of activated charcoal (Darco (1-60) and 2.0 kg of diatomaceous earth
is packed as a thick slurry in a 6 inch column. The pH of the water is 5.2-5.5.
With this bed, a head of 4 feet of solvent is necessary to achieve a suitable
flow rate. After packing, the column is washed with water for several hours to
settle and remove solubles. The solution is applied to the column with positive
pressure equivalent to a head of four foot of water. One retention volume of 9
L of water is then applied to the column. This is followed by a 5% acetone
solution. The total solvent flow is 36 L. The colorless eluate is discarded. The
elution front which is easily detected is a light yellowish-green solution. This
solution is retained. The solution is concentrated by vacuum distillation until a
concentration of 20-25 mg/ml is reached. This solution is applied to a column
prepared in an identical manner as described herein and treated by the same
procedure as the primary adsorption. The percolate is concentrated by
vacuum distillation until a concentration of 60-75 mg/ml is reached. The
quantity of solution is now approximately 300 ml. Absolute alcohol (450 ml) is
added. The solution is gently warmed to complete solution and then stored at
5°C for several hours. The O-diazoacetyl-L-serine which separates in
crystalline form is collected and purified by recrystallization from 60-70%
ethanol, is an aqueous buffer of pH 7.
Therapeutic Function
Antineoplastic, Antifungal
General Description
Pale yellow to green crystals. Used as an antifungal agent.
Air & Water Reactions
Very soluble in water.
Reactivity Profile
AZASERINE is incompatible with acids.
Hazard
Toxic; possible carcinogen; neoplastigenic; tumorigenic; poison; teratogen; mutagen.
Biological Activity
azaserine, as a naturally occurring serine derivative diazo compound, functions as a purine antagonist and structural analogue of glutamine that inhibits enzymatic activities involving in the pathways of glutamine metabolism. azaserine, an antibiotic and antitumor agent, is used as a potential antineoplastic agent in clinical studies. azaserine dampens the biosynthesis of purine via reacting with cysteine residues in the enzyme active sites. in addition, azaserine triggers dna damage by the formation of carboxymethylated bases and o6-methylguanine.
Safety Profile
Suspected carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Poison by ingestion, intraperitoneal, and subcutaneous routes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
in vitro
azaserine showed cytotoxicity in raji cells, which was partly due to inhibition of de novo purine biosynthesis, and the expression of o6-methylguanine-dna methyltransferase did not provide protection against cell killing, suggesting that o6-methylguanine was not a major contributor to the cytotoxic dna damage triggered by azaserine. azaserine killed the raji hypoxanthine-guanine phosphoribosyltransferas-deficient(hprt-) mex- cells. in contrast, the raji hprt+ mex- cells were more resistant to azaserine. additionally, azaserine blocked the growth of raji hprt+ mex-cells when treated with 300 μm [1].
in vivo
cd-l mice and w/lew rats were injected intraperitoneally with azaserine at a dose of 10 mg/kg body weight once a week for 5 weeks. after 6 months, compared to the control rats and mice, the azaserine-treated animals had a slightly higher incidence of pancreatic atypical acinar cell nodules (aacn) and the average size of aacn of azaserine-treated animals was larger. in addition, the concentration of [14c] azaserine and/or its metabolites was lower in mouse pancreas than in rat pancreas [2].
IC 50
7 μm: inhibits parasite growth.
Purification Methods
Crystallise azaserine from 90% EtOH. Also dissolve it in H2O, filter it through Supercel and add EtOH to give azaserine as pale yellow crystals. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 1 pp 75-76 1961, Curphey & David J Org Chem 43 4666 1978, Beilstein 4 IV 3124.]
References
[1]. o'driscoll, m., macpherson, p., xu, y., & karran, p. the cytotoxicity of dna carboxymethylation and methylation by the model carboxymethylating agent azaserine in human cells. carcinogenesis. 1999; 20(9): 1855-1862.
[2]. b. d. roebuck, herman s. lilja, thomas j. curphey, daniel s. longnecker; pathologic and biochemical effects of azaserine in inbred wistar/lewis rats and noninbred cd-1 mice. j natl cancer inst. 1980; 65 (2): 383-389.
AZASERINE Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9341 | 55 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 27792 | 58 |
| BOC Sciences | 16314854226; +16314854226 | inquiry@bocsci.com | United States | 19743 | 58 |
| Nantong HI-FUTURE Biology Co., Ltd. | +undefined18051384581 | sales@chemhifuture.com | China | 3136 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 19949 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33350 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15848 | 69 |
115-02-6(AZASERINE)Related Search:
1of3