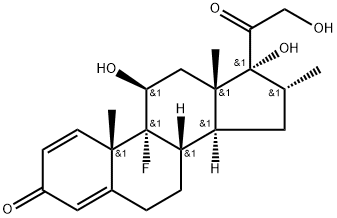
덱사메타손
|
|
덱사메타손 속성
- 녹는점
- 262-264 °C (lit.)
- 끓는 점
- 568.2±50.0 °C(Predicted)
- 알파
- 75 º (c=1, dioxane)
- 밀도
- 1.1283 (estimate)
- 굴절률
- 76 ° (C=1, Dioxane)
- 인화점
- 9℃
- 저장 조건
- 2-8°C
- 용해도
- 에탄올: 1mg/mL
- 산도 계수 (pKa)
- 12.13±0.70(Predicted)
- 물리적 상태
- 가루
- 색상
- 회백색
- 수용성
- 10mg/100mL(25℃)
- 감도
- Light Sensitive
- Merck
- 14,2943
- BRN
- 2066651
- BCS Class
- 1 (CLogP), 3 (LogP)
- 안정성
- 안정적인. 타기 쉬운. 강산화제, 강염기, 산무수물, 산염화물과 혼합할 수 없습니다. 빛에 민감 할 수 있습니다.
- InChIKey
- UREBDLICKHMUKA-CXSFZGCWSA-N
- CAS 데이터베이스
- 50-02-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
| 위험품 표기 | Xi,Xn,T,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 43-40-36/37/38-20/21/22-42/43-39/23/24/25-23/24/25-11 | ||
| 안전지침서 | 36/37-45-36-26-22-16 | ||
| 유엔번호(UN No.) | UN1230 - class 3 - PG 2 - Methanol, solution | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | TU3980000 | ||
| TSCA | Yes | ||
| HS 번호 | 29372210 | ||
| 유해 물질 데이터 | 50-02-2(Hazardous Substances Data) | ||
| 독성 | LD50 oral in rat: > 3gm/kg | ||
| 기존화학 물질 | KE-17063 |
덱사메타손 C화학적 특성, 용도, 생산
개요
The activity of dexamethasone, as measured by glycogen deposition, is 20 times greater than that of hydrocortisone. It has five times the anti-inflammatory activity of prednisolone. Clinical data indicate that this compound has seven times the antirheumatic potency of prednisolone. It is roughly 30 times more potent than hydrocortisone. Its pharmacokinetics are presented in Table 33.3. Routes of metabolism for dexamethasone are similar to those for prednisolone, with its primary 6β-hydroxy metabolite being recovered in urine. Dexamethasone sodium phosphate is the water-soluble sodium salt of the 21-phosphate ester, with an IV half-life of less than 10 minutes because of rapid hydrolysis by plasma phosphatases. Peak plasma levels for dexamethasone usually are attained in approximately 10 to 20 minutes following its IV administered dose. A similar reaction occurs when the phosphate ester is applied topically or by inhalation.화학적 성질
White or almost white, crystalline powder.용도
Dexamethasone is used for the same indications as all corticosteroids; however, it exhibits a significantly more powerful anti-inflammatory and anti-allergic action. It is used for circulatory collapse—shock during or after surgical operations, trauma, blood loss, myocardial infarction, and burns. It is also used in severe infections—toxemia, vascular collapse in meningococcosis, septicemia, diphtheria, typhoid fever, and peritonitis. It is used in severe allergic conditions—asthmatic status, laryngeal edema, severe anaphylactic reactions to medicinal drugs, and pyrogenic reactions.Indications
Cushing’s disease is defined as hypercortisolism due to chronic overproduction of corticotrophin by a corticotroph adenoma. Cortisol’s lack of suppressibility during the administration of low doses of dexamethasone but suppressibility during high-dose dexamethasone is the key diagnostic finding in 99% of the patients with Cushing’s disease. This contrasts with the lack of glucocorticoid suppressibility typically found in patients with corticotrophin-independent hypercortisolism (Cushing’s syndrome). A judicious selection of the available tests may be necessary to obtain an accurate diagnosis in patients with Cushing’s syndrome.일반 설명
Dexamethasone, 9-fluoro-11β,17,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione,is the 16 -isomer of betamethasone.Dexamethasone acetate, USP (21-acetate)
Dexamethasone sodium phosphate, USP (21-sodiumphosphate).
공기와 물의 반응
Insoluble in water.반응 프로필
Dexamethasone may be sensitive to prolonged exposure to light. Dexamethasone is incompatible with strong oxidizers, strong acids, acid chlorides and acid anhydrides. Oxidation may occur with bases.화재위험
Flash point data for Dexamethasone are not available; however, Dexamethasone is probably combustible.생물학적 활성
Glucocorticoid; anti-inflammatory. Reduces levels of activated NF- κ B in immature dendritic cells (DCs) and inhibits differentiation into mature DCs.Pharmacology
Dexamethasone is a corticosteroid with high glucocorticoid activity and virtually no mineralocorticoid activity. I ts mechanism of action as an antiemetic is unknown, but it is possible that either direct genomic or indirect non-genomic effects on 5-HT3 and GABAA receptors contribute to its antiemetic activity. Many of the original studies were carried out using 8– 10mg of dexamethasone phosphate, but smaller doses (2.5–4mg) provide equal antiemetic efficacy with minimal risk of adverse effects. Concerns relating to adrenal suppression and other steroid-induced adverse effects (including increased risk of bleeding) after a single dose of dexamethasone remain largely unfounded. O ne of the most unpleasant adverse effects of dexamethasone involves intense perineal stimulation after rapid i.v. injection.Pharmacokinetics
The activity of dexamethasone, as measured by glycogen deposition, is 20 times greater than that of hydrocortisone. It has five times the anti-inflammatory activity of prednisolone. Clinical data indicate that this compound has seven times the antirheumatic potency of prednisolone. It is roughly 30 times more potent than hydrocortisone. Its pharmacokinetics are presented in Table 33.3. Routes of metabolism for dexamethasone are similar to those for prednisolone, with its primary 6β-hydroxy metabolite being recovered in urine. Dexamethasone sodium phosphate is the water-soluble sodium salt of the 21-phosphate ester, with an IV half-life of less than 10 minutes because of rapid hydrolysis by plasma phosphatases. Peak plasma levels for dexamethasone usually are attained in approximately 10 to 20 minutes following its IV administered dose. A similar reaction occurs when the phosphate ester is applied topically or by inhalation.Safety Profile
Poison by intraperitoneal and subcutaneous routes. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of F-.Purification Methods
Dexamethasone has been recrystallised from Et2O or small volumes of EtOAc. Its solubility in H2O is 10 mg/100mL at 25o; and is freely soluble in Me2CO, EtOH and CHCl3. [Arth et al. J Am Chem Soc 80 3161 1958; for the -methyl isomer see Taub et al. J Am Chem Soc 82 4025 1960, see Beilstein 8 IV 3501.]덱사메타손 준비 용품 및 원자재
원자재
준비 용품
덱사메타손 공급 업체
글로벌( 769)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49391 | 58 |
| Shenzhen Monkono Technology Co.,Ltd | +86-17063441314 |
sansbiotech@outlook.com | China | 677 | 58 |
| Anhui Yisheng Technology Co., LTD | +86-19355901310 +86-19355901310 |
alice@ah-yisheng.com | China | 333 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 |
xie@china-sinoway.com | China | 992 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 |
admin01@hsnm.com.cn | China | 2118 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 1000 | 58 |
| Henan Suikang Pharmaceutical Co.,Ltd. | +86-18239973690 +86-18239973690 |
sales@suikangpharm.com | China | 186 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21689 | 55 |
덱사메타손 관련 검색:
플루오린(불소) 덱사메타손 아세트산, 무수물 덱사메타손 나트륨 인산염 프레드니손 덱사메타손
Dexamethasone palmitate
Betamethasone EP Impurity D
Betamethasone-17-ketone
9β,11β-Epoxy-17,21-dihydroxy-16β-methylpregna-1,4-diene-3,20-dione
Clobetasol
17,21-dihydroxy-16beta-methylpregna-1,4,9(11)-triene-3,20-dione
Halometasone
Flumethasone-17-acetate
DEXAMETHASONE SODIUM PHOSPHATE
Alclometasone
Dexamethasone sodium phosphate
Flumethasone 21-pivalate
16α-methyl Epoxide 21-Acetate